Projected effects of climate change on vector-borne zoonotic diseases of animals

Summary
Environmental temperature change of climate and rainfall distribution and intensity, the migration of wild animals, and movement of domestic animals and the migration of people and increasing tourism are all affecting the distribution and abundance of insect, arachnid and molluscan vectors of disease. Thesustained control of the insect vectors of dengue and leishmaniasis is difficult because their high reproductive potential allows the vector populations to recover quickly after intervention given adequate breeding conditions. By contrast, tsetse flies, the vectors of trypanosomiasis, have a lower reproductive potential and could be eliminated over large areas, given adequate organization and surveillance. There are several developments which should be implemented. A number of actions and policy changes are proposed. These include: improved advice to local farmers, and the use of more appropriate vaccines and other medicines, the prevention of the sale of OTC antibiotics, greater knowledge of the ecology of vectors, the GM/gene editing of mosquitoesand the use of remote sensing devices.
Glossary: : Zoonotic disease; Pertaining to a zoonosis: a disease that normally exists in animals that can be transmitted to and infect people, or that can be transferred from people to animals and infect them.
The earth’s temperature has risen about 0.8o C since 1880. It has been estimated that 20-30% of all vertebrate animals would become extinct if the average temperature rises by the 2-3 ° C anticipated this century1.
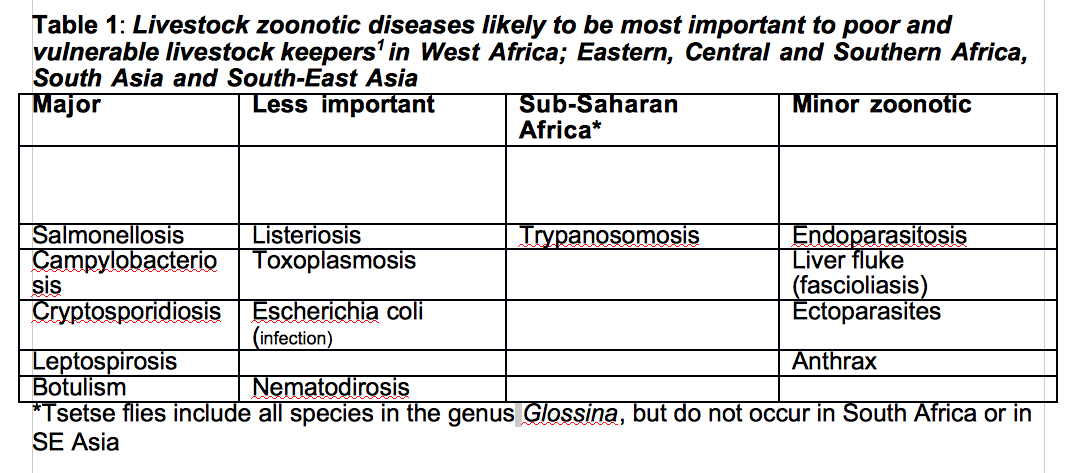
The distribution and intensity of rainfall is also changing, leading, for example, to increased frequency of droughts and floods in India2. The effect is that the incidence, especially of vector-borne animal disease is likely to rise. Climate change is resulting in emergence and re-emergence of a plethora of infectious, mostly zoonotic, diseases throughout the world. Around 75% of the emerging animal diseases are zoonotic in nature 1,2,3(Table 1) with wildlife being one of the major sources of infection4.
The livestock sector is extremely important to rural livelihoods and the global economy. In 2013, there were an estimated 38 billion livestock in the world, or nearly five animals for every person. Most (>80%) were in developing countries and around one billion poor farmers keep livestock1.
The burden of animal disease in developing countries is high: disease probably kills 20% of ruminants and more than 50% of poultry each year, causing a loss of approximately US$ 300 billion. Climate change can exacerbate these losses as, of 65 animal diseases identified as most important to poor people, over half are climate sensitive1.
Vectors: arthropods (insects and arachnids), rodents, Molluscan and domestic and wild animals are all capable of being vectors of disease and are especially sensitive to climate change. Changes in rainfall and temperature regimes may affect both the distribution and the abundance of disease vectors, as can changes in the frequency of extreme events. Infectious diseases are transmitted by arthropods and rodents and also are disseminated by water, food, and air. Arthropod vectors tend to be more active at higher temperatures, when they need to feed more regularly to sustain the increase in their metabolic rate. This enhances the chances of infections being transmitted between hosts so that small changes in vector characteristics can produce substantial changes in disease1. For example, there is increased research activity in searching for better control of the three diseases caused by the trypanosomatids, the related kinetoplastid pathogens: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi . These protozoa cause some of the most debilitating diseases of humans: cutaneous leishmaniasis, African Sleeping Sickness, and Chagas disease.
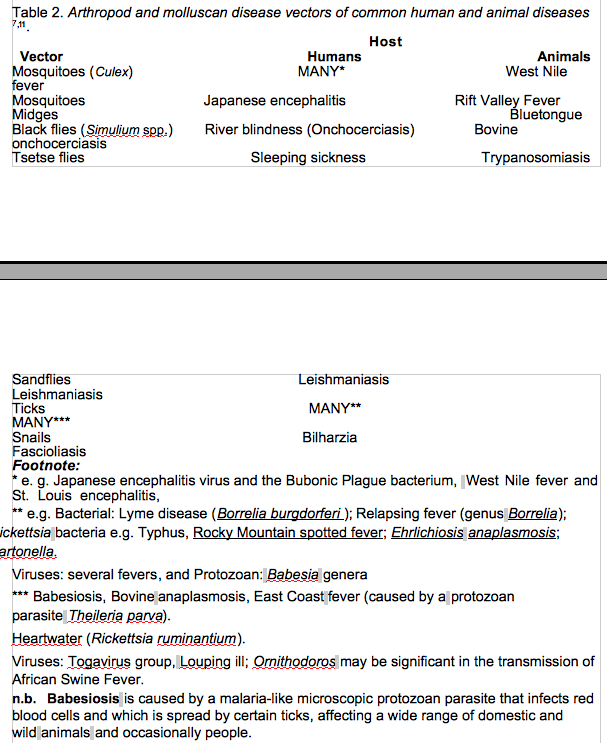
These diseases possess complex life cycles that involve development in mammalian and insect hosts5,6although they have dissimilar geographical distributions. This reflects their different insect vector characteristics and range of vector contact with humans. These diseases disproportionately afflict poor and remote populations with limited access to health services. Moreover, their pathogenic mechanisms are poorly understood, but typically entail immunological processes, so that herd immunization reduces the number of susceptible animals in a population and augments herd immunity making infection more difficult to spread4. Increased environmental temperature both increases the rate of development of the leishmania parasite and the activity the sandfly vector. The vector takes more frequent blood meals, which in turn enhances transmission (Table 2)7. Higher temperatures also increase the developmental rate of ticks and their over-winter survival rate is improved. This will increase the risk of, for example, Lyme Disease spread8(Table 2).
The major viral diseases with arthropod vectors (often termed the arboviral diseases), including dengue fever, Rift Valley fever and West Nile virus; Lyme disease (caused by Borreliabacteria); malaria (caused by the Plasmodium parasite); rodent vector diseases such as bubonic plague (caused by Yersinia pestisbacteria) and hantavirus pulmonary syndrome have all been shown to have a distinct seasonal pattern and in some instances their frequency has been shown to be weather sensitive9.
Despite rural livestock keepers having a basic understanding of zoonotic disease risk, several factors, such as the handling and consumption of sick and dead animals and purchasing medications for sick animals ‘over the counter’ (OTC) potentially increase the risk of zoonotic disease transmission as well as the development and spread of antimicrobial resistance10.
The spread of bluetongue (Orbivirus) and its vectors (biting midges of theCulicoides genus) presents some of the strongest evidence to date that climate change is driving vector-borne diseases into new regions, as warming and disease spread have occurred at the same times in the same places7.
Sustained control of the insect vectors is difficult for dengue and leishmaniasis because their high reproductive potential allows the vector populations to recover quickly after intervention wherever adequate breeding conditions exist. By contrast, tsetse flies, the vectors for Sleeping Sickness, have a much lower reproductive potential and could be eliminated over large areas, given adequate organization and surveillance12.
Through the African Union, African nations are developing a large-scale initiative for area-wide elimination of tsetse flies, partly because of Sleeping Sickness, but also because of their importance as vectors of animal trypanosomiasis, which poses a serious constraint to livestock development and agriculture13.
Causes of increased frequency of zoonotic diseases:
-
Climate change: temperature, regional drought distribution and intensity of precipitation (leading to flooding) 9.
-
Change in: farming practices; social, cultural, religious and lifestyle habits.
-
Rapid population growth and intensification of animal production.
-
Poverty and lack of local advice, increased numbers of animals and humans with impaired host defences.
-
Animal migration and more frequent movement across international boundaries by tourists, workers, immigrants, and refugees.
Action and Policy changes required:
-
Better understanding of the environmental conditions (landscape + climate) that control vectors. Spatially link environmental conditions to the vector distribution.
-
Remote sensing of data + Geographic Information Systems (GIS) which characterizes the environment: vegetation, altitude, hydrology, climate7,11.
-
Correct advice to local farmers. Recognition thatinfection is spread by movement of exposed and infected animals and diffusion of infected vectors.
-
Vectors: knowledge of adoption of climate change in habitat control. Competent use of insecticides for control of vector reservoirs8.Prevention of indiscriminate use of ‘over the counter’ antibiotics and other antimicrobials. There is also current research into finding an alternative control method by using genetic engineering or editing to produce mutant mosquitoes incapable of transmitting the disease that will be self-propagating when introduced into wild populations - referred to as ‘gene-drive’ control technology.
5) Development, use and distribution of more appropriate, cost-effective and disease specific vaccines and other treatments are necessary, e.g. West Nile virus and Leishmaniasis vaccines.
6) Assess changes in the way that food is produced, processed and distributed. Therefore, collaboration between human medical and veterinary scientists, public health practitioners and laboratory scientists is essential in order to investigate new and emerging zoonotic diseases.
7) Investigations by epidemiologists, ecologists, and environmentalists are crucial in establishing patterns to prevent outbreaks of zoonotic diseases to determine the directional interactions of transmission between wildlife, domestic animals and humans
8) Fully equipped laboratories that contain subtyping technique tools are essential to detect disease outbreaks and characterize transmission routes. By using molecular subtyping, different strains can be differentiated based on their genotypes and phenotypes.
|
9) Measures of the morbidity, mortality, and economic burden of disease in animals and humans. 10) Conduct cost-benefit and effectiveness analyses of interventions for zoonotic infections13. 11) Prevention of movement of infected livestock from endemic areas to non-endemic countries (e.g. Rift Valley Fever8) to avoid regional spread of the disease if patent vectors and reservoirs are also present. Thanks: I would like to thank David Cooper and Professor Chris Gaskell for their helpful advice on reading this paper. |
References
1. Grace D, Bett B, Lindahl J, Robinson T. 2015. Climate and livestock disease: assessing the vulnerability of agricultural systems to livestock pests under climate change scenarios. CCAFS Working Paper no. 116. Copenhagen, Denmark: CGIAR Research Program on Climate Change, Agriculture and Food Security (CCAFS).: www.ccafs.cgiar.orghttp://hdl.handle.net/10568/66595
2. Ashaq Ashraf, Mohammad Maqbool Darzi, Basharat Maqbool Wani, Showkat Ahmad Shah, Mir Shabir and Majid Shafi (2017) Climate change and infectious diseases of animals: A review. Journal of Entomology and Zoology Studies; 5(5): 1470-1477
3. 34. Taylor LH. (2001) Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci.; 356:983-989.
4. 35. Dubal ZB, Barbuddhe SB, Singh NP. (2014) Important Zoonotic Diseases: Prevention and control. Technical Bulletin No. 39. ICAR Research Complex for Goa (Indian Council of Agricultural Research), Old Goa- 403 402, Goa, India.
5. Rogers, D.J. (1988)The dynamics of vector-transmitted diseases in human communities. Philosophical Transactions of the Royal Society B. 31 October;321, issue 1207. DOI: 10.1098/rstb.1988.0106.
6.Marilyn Parsons Elizabeth A Worthey, Pauline N Ward and Jeremy C Mottram (2005)Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics, 6:127. https://doi.org/10.1186/1471-2164-6-127.
7. Jan van Dijk & Matthew Baylis LUCINDA: Liverpool University Climate and Infectious Diseases of Animals. Predicting the effects of climate change on infectious diseases of animals Veterinary Clinical Science, Leahurst, Liverpoolwww.liv.ac.uk/vetepi
8. Preneshni R. Naicker (2011) The impact of climate change and other factors on zoonotic diseases. ARCHIVES OF CLINICAL MICROBIOLOGY, 2No. 2:4 doi: 10:3823/226.
9. Neelam Sachan and V.P.Singh (2010) Effect of climatic changes on the prevalence of zoonotic diseases. Veterinary World, 11:519-522.
10. Christopher Lowenstein, William F. Waters, Amira Roess, Jessica H. Leibler, and Jay P. Graham(2016). Animal Husbandry Practices and Perceptions of Zoonotic Infectious Disease Risks among Livestock Keepers in a Rural Parish of Quito, Ecuador. Am J Trop Med Hyg. Dec 7; 95(6): 1450–1458. doi: 10.4269/ajtmh.16-0485.
11. Kalluri S, Gilruth P, Rogers D, Szczur M (2007) Surveillance of Arthropod Vector-Borne Infectious Diseases Using Remote Sensing Techniques: A Review. PLoS Pathog 3 (10) : e 116.https://doi.org/journal.ppat.0030116).
12. P. Cattand, P. Desjeux, M. G. Guzmán, J. Jannin, A. Kroeger, A. Medici, P. Musgrove, M. B. Nathan, A. Shaw, and C. J. Schofield. (2006) Disease Control Priorities in Developing Countries. 2nd edition. Chapter 23Tropical Diseases Lacking Adequate Control Measures: Dengue, Leishmaniasis, and African Trypanosomiasis. Jamison DT, Breman JG, Measham AR, et al.,eds. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; New York: Oxford University Press;
13. Nitin Sekar, Naman K. Shah, Syed Shahid Abbas, Manish Kakkar(2011) Research Options for Controlling Zoonotic Disease in India, 2010–2015. PLoS One. 6(2): e17120.Published online 2011 Feb 25. doi: 10.1371/journal.pone.0017120A https://earthobservatory.nasa.gov/WorldOfChange/decadaltemp.php
Figures